Physics for Electronics Engineering: Unit III: Semiconductors and Transport Physics
Semiconductors
Definitions, Properties, Classification, Example, Characteristics, Uses
Semiconducting material has electrical conductivity between a good conductor and a good insulator. It is simply called semiconductor. It is a special class of material which is very small in size and sensitive to heat, light and electricity.
Semiconductors and Transport Physics
Introduction
Semiconducting
material has electrical conductivity between a good conductor and a good
insulator. It is simply called semiconductor. It is a special class of material
which is very small in size and sensitive to heat, light and electricity.
Semiconducting
materials behave as insulators at low temperature and as conductors at high
temperature. Moreover, these materials have two types of charge carriers i.e.,
electrons and holes.
Germanium
and silicon are two important elemental semiconductors. They are used in diodes
and transistors.
Gallium
arsenide (GaAs) and indium phosphide (InP) are two important compound
semiconductors. They are used in LEDs and Laser diodes.
The
study of semiconducting materials is essential for engineers due to their wide
applications in semiconductor devices in engineering and technology
The
invention of semiconductors opened a new branch of technology called solid
state electronics.
It leads to the ICs, Microprocessors, computers and supercomputers.
In
short, semiconductors play a vital role in almost all advanced electronic
devices.
Definition
Based
on Electrical resistance
Semiconductor
has electrical resistance which is lesser than an insulator but more than that
of a
conductor.
Its electrical resistivity is in the order of 10-4 to 0.5 ohm metre.
Based
on Energy band
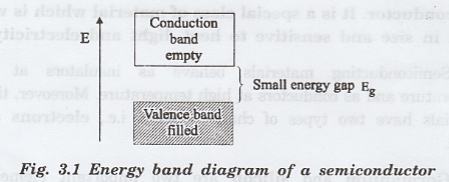
A
semiconductor has nearly an empty conduction band and almost filled valence
band with a very small energy gap (~ 1 eV). (Fig 3.1)
General
properties of the semiconductors
i.
They have crystalline structure.
ii.
Bonding between the atoms is formed by covalent bond.
iii.
They have empty conduction band at OK.
iv.
They have almost filled valence band.
v.
The energy gap is small.
vi. They exhibit a negative temperature coefficient of resistance. ie., increase in temperature leads to decrease in resistance.
vii.
If impurities are added to a semiconductor, its electrical conductivity
increases. Further, if temperature of the semiconductor is increased, its
electrical conductivity increases.
This
property is in contrary to that of metals in which if temperature is increased
or impurities are added, their electrical conductivity decreases.
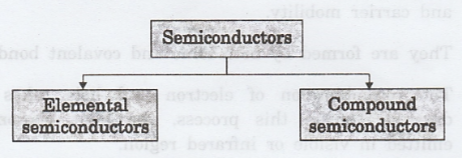
Classification
of semiconductors
The
semiconductors are classified mainly into two types based on the composition of
materials. They are
(i)
Elemental semiconductors
(ii)
Compound semiconductors
Elemental Semiconductors
(Indirect
band gap semiconductor)
The
semiconductors which are made from a single element of fourth group elements in
periodic table are known as elemental semiconductors.
They
are also called as indirect band gap semiconductors.
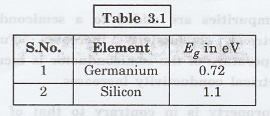
Example
Two important elemental semiconductors and their energy band gaps are given in table 3.1.
Compound
semiconductors
(Direct
band gap semiconductors)
Semiconductors
which are formed by combining third and fifth group or second and sixth group
elements in the periodic table are known as compound semiconductors.
They
are also called as direct bandgap semiconductors.
Characteristics
i.
The compound semiconductor have large forbidden gap and carrier mobility.
ii.
They are formed by both ionic and covalent bonds.
iii.
The recombination of electron and hole takes place directly. During this
process, the light photons are emitted in visible or infrared region.
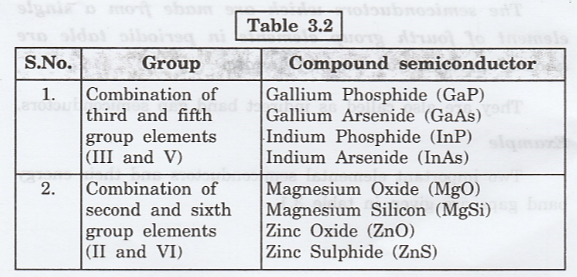
Some
important compound semiconductors are given table 3.2.
Uses
The
compound semiconductors are used in photovoltaic cell, photoconductive cell,
LEDs [Light Emitting Diode] and Laser.
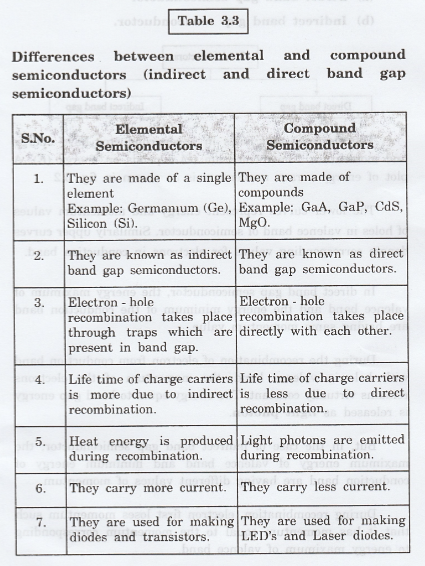
Differences
between elemental and compound semiconductors (indirect and direct band gap
semiconductors)
Physics for Electronics Engineering: Unit III: Semiconductors and Transport Physics : Tag: : Definitions, Properties, Classification, Example, Characteristics, Uses - Semiconductors
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
