Physics for Electronics Engineering: Unit II: Electrical and Magnetic Properties of Materials
Paramagnetism in the Conduction Electrons in Metals
Derivation
According to Langevin's theory the paramagnetic susceptibility is inversely proportional to the temperature. However, some metals have been found to exhibit paramagnetism independent of temperature.
PARAMAGNETISM IN THE CONDUCTION ELECTRONS
IN METALS
According
to Langevin's theory the paramagnetic susceptibility is inversely proportional
to the temperature. However, some metals have been found to exhibit
paramagnetism independent of temperature.
It was. W. Pauli (1927) who demonstrated that this is due to paramagnetism of free electrons (that constitute the electron gas), since they can orient only in two directions, either along the magnetic field or against it.
In
order of tog understand the existence of Pauli paramagnetism, let us recall the
curve between density of states versus energy (Fig. 2.27) at absolute zero of
temperature. That curve may be split into two parts with spins pointing in the
+ve z-direction and other with spin in the opposite direction, as shown in Fig.
2.27(a).
In
the absence of an external magnetic field, the distribution of electrons with
spins parallel to z-direction is equal to the number of electrons with opposite
spins and hence the net magnetic moment of the electron gas is zero.
When
a magnetic field (B) is applied along the z-direction, the energy of the spins
aligned parallel to B is lowered by the amount μB' while the energy of the
spins opposite to B is raised by the same amount (Fig. 2.27(b)).
As
a result of this, the Fermi level for the two spin distributions shift with
respect to each other and give rise to energetically unstable situation.
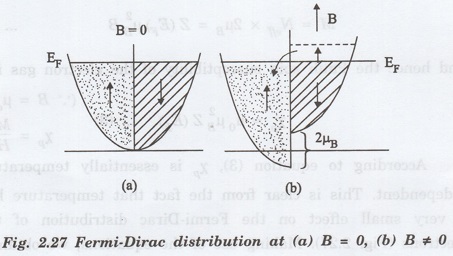
In
order to acquire the stable configuration, the electrons lying near the Fermi
level with antiparallel spins flip into the region of parallel spins until the
two Fermi levels become equal s become again (Fig 2.27(b)).
The
number of electrons which effectively change their direction is equal to the
density of states at the energy level (Z (EF)) in one of the spin
distribution times the change in energy, i.e.
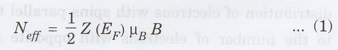
where
the factor 1/2 is due to the fact that the density of states of one spin
distribution is half of the total density of states. μB is magnetic
moment of electron.
Thus
after the application of the field, the number of electrons with spins parallel
to the field is greater than the electrons with opposite spin by Neff,
to a net magnetization.
Since
each flip increases the magnetization by 2 μB (from -μB
to +μB), the net magnetization is given by
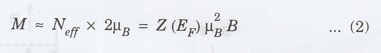
and
hence the Pauli spin susceptibility of the electron gas is

According to equation (3), χP, is essentially temperature independent. This is clear from the fact that temperature has a very small effect on the Fermi-Dirac distribution of the electrons (Fig. 2.27). Making use of the equations, we obtain
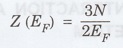
N
- No. of electrons per unit volume.
so
that (3) becomes,
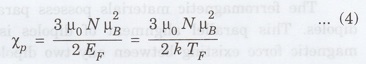
where EF = kTF. This
equation can be rewritten in terms of the classical susceptibility as

where 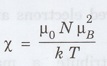
Since
TF is normally very high, χp, is smaller than χ by about
two orders of magnitude, which is in agreement with the experimental results.
In transition metals, the paramagnetic susceptibility, χp is
exceptionally high, because Z (EF) is large.
Physics for Electronics Engineering: Unit II: Electrical and Magnetic Properties of Materials : Tag: : Derivation - Paramagnetism in the Conduction Electrons in Metals
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
