Physics for Electronics Engineering: Unit III: Semiconductors and Transport Physics
Intrinsic Semiconductors and Energy Band Diagram
A semiconductor in extremely pure form is known as intrinsic semiconductor. Its electrical conductivity is changed only by thermal excitation.
INTRINSIC SEMICONDUCTORS
A
semiconductor in extremely pure form is known as intrinsic semiconductor. Its
electrical conductivity is changed only by thermal excitation.
The
common examples for intrinsic semiconductors are pure silicon (Si) and
germanium (Ge). They belong to fourth group elements in the periodic table.
Germanium ha 32 electrons and silicon has 14 electrons in their atomic
structures.
They
are tetravalent atoms since they have four valence electrons. The neighbouring
atoms form covalent bonds by sharing four electrons with each other so as to
form a stable structure.
ENERGY BAND DIAGRAM
Fig.
3.3 shows a two-dimensional crystal structure of germanium and energy band
representation semiconductor at very low temperature.
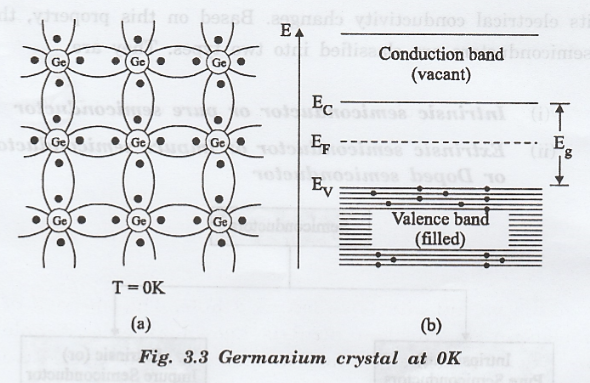
Fig. 3.3(a) Two-dimensional
representation of germanium solid. No free electron is available as all the
valence electrons are engaged in covalent bonds.
Fig. 3.3(b) Energy band
representation. Valence band is fully occupied and conduction band is
completely vacant.
At
very low temperature say OK, no free electrons are available for conduction.
Hence, this semiconductor behaves as an insulator at very low temperature.
Charge
carriers in intrinsic semiconductor
To
get free electrons, covalent bonds must be broken. There are many ways of
breaking covalent bond and setting the electrons free. One such way is to
increase crystal temperature above OK.
When
the temperature of intrinsic semiconductor is increased, some of the electrons
get sufficient energy to break covalent bonds.
Once
the electrons are liberated from bond, they become free electrons. These free
electrons move randomly through crystal. (Fig. 3.4(a))
As
shown in fig. 3.4 (b), the energy required to break a covalent bond and to set
an electron free is equal to band gap energy E. It is about 0.72 eV for
germanium and 1.1 eV for silicon.
When
an electron acquires energy E, it jumps from valence band to conduction band.
As a result, a vacant site (empty space) is created in valence band.
This vacant site is called as a hole. The absence of an electron in covalent bond is known as hole. A hole can attract an electron and hence it acts as a positive charge.
When
an electrical field is applied, these free electrons acquire directional motion
and contribute to electrical conductivity.
For
every electron freed from covalent bond, one hole is created in the crystal. It
is relatively easy for a valence electron in a neighbouring atom to leave its
covalent bond and fill this hole.
As
a result, an electron moving from a covalent bond to fill a hole leaves behind
a hole in its original position.
The
hole effectively moves in a direction opposite to that of an electron. The hole
in its new position may now be filled by an electron from another covalent
bond.
Thus
hole will correspondingly move one more step in the direction opposite to the
motion of the electron.

(a) Thermal vibrations of atoms
lead to breaking up of covalent bonds. Consequently, a free electron and a
vacancy are produced simultaneously.
(b) Energy band representation.
Energy Eg (= Ec – Ev) causes transition of
electrons from valence band to conduction band, leaving vacancies (hole) behind.
Therefore,
in intrinsic semiconductor, current conduction is due to the movement of both
electrons and holes.
Here,
the number of electrons is equal to the number of holes at any given
temperature.
Physics for Electronics Engineering: Unit III: Semiconductors and Transport Physics : Tag: : - Intrinsic Semiconductors and Energy Band Diagram
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
