Physics for Electronics Engineering: Unit II: Electrical and Magnetic Properties of Materials
Quantum Free Electron (QFE) Theory
Definition, Postulates, Merits, Demerits, Example, Equation
The failures of classical free electron theory were rectified in quantum theory given by Sommerfeld in the year 1928. This theory uses quantum concepts and hence it is known as quantum free electron theory. Sommerfeld used Schrodinger's wave equation de-Broglie's concept of matter waves to obtain the expression for electron energies. He approached the problem quantum mechanically using Fermi - Dirac statistics instead of classical Maxwell - Boltzmann statistics.
QUANTUM FREE ELECTRON (QFE) THEORY
The
failures of classical free electron theory were rectified in quantum theory
given by Sommerfeld in the year 1928.
This
theory uses quantum concepts and hence it is known as quantum free electron
theory.
Sommerfeld
used Schrodinger's wave equation de-Broglie's concept of matter waves to obtain
the expression for electron energies.
He
approached the problem quantum mechanically using Fermi - Dirac statistics
instead of classical Maxwell - Boltzmann statistics.
Postulates of Quantum Free Electron
Theory
i.
The potential energy of an electron is uniform or constant within the metal.
ii.
The electrons have wave nature.
iii. The allowed energy levels of an electron are quantized.
iv.
The electrons move freely within the metal and they are not allowed to leave
the metal due to existance of potential barrier at its surfaces.
v.
The free electrons obey Fermi - Dirac statistics.
Merits of Quantum Free Electron
Theory
i.
This theory treats the electron quantum mechanically rather than classically.
ii.
It explains the electrical conductivity, thermal conductivity, specific heat
capacity of metals, photoelectric effect and Compton effect, etc.
Demerits of Quantum Free Electron
Theory
i.
Even though it explains most of the physical properties of the metals, it fails
to state the difference between conductor, semiconductor and insulator.
ii.
It also fails to explain the positive value of Hall coefficient and some of the
transport properties of the metals.
Electrons in Metals - Particle in a
three dimensional box
The
solution of one-dimensional potential well is extended for a three-dimensional
potential box.
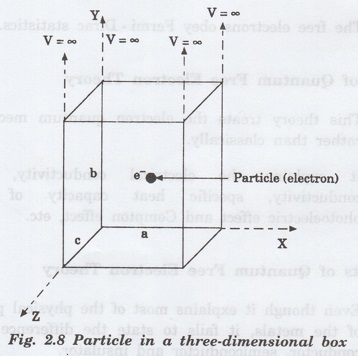
In
a three-dimensional potential box, the particle (electron) can move in any
direction in space. Therefore, instead of one quantum number n, we have to use
three quantum numbers, nx, ny and nz,
corresponding to the three coordinate axes namely x, y and z respectively.
If
a, b, c are the lengths of the box as shown in figure 2.8 along x, y and z
axes, then
Energy
of the particle = Ex + Ey + Ez
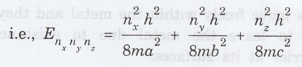
If
a = b = c as for a cubical box, the
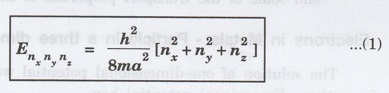
The
corresponding normalised wave function of an electron in a cubical box may be
written as
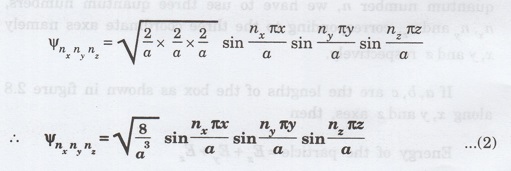
From
the equations (1) and (2), we understand that several combinations of the three
quantum numbers (nx, ny and nz) lead to
different energy eigen values and eigen functions.
Example
Suppose
a state has quantum numbers, then
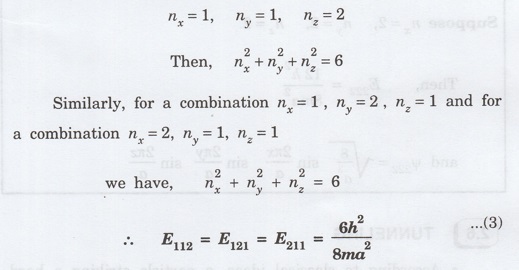
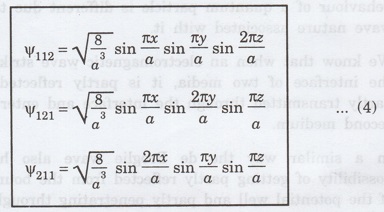
The
corresponding wave functions are written as
Physics for Electronics Engineering: Unit II: Electrical and Magnetic Properties of Materials : Tag: : Definition, Postulates, Merits, Demerits, Example, Equation - Quantum Free Electron (QFE) Theory
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
