Physics for Electronics Engineering: Unit II: Electrical and Magnetic Properties of Materials
Ferromagnetic Effect
Exchange Interaction and Ferromagnetism
Certain metals like iron (Fe), cobalt (Co), nickel (Ni) and certain alloys exhibit high degree of magnetisation. These materials show the spontaneous magnetization i.e., they have magnetisation (atomic magnetic moments are aligned) even in the absence of an external magnetic field.
FERROMAGNETIC EFFECT
Certain metals like iron (Fe), cobalt (Co), nickel (Ni) and certain alloys exhibit high degree of magnetisation.
These materials show the spontaneous magnetization i.e., they have magnetisation (atomic magnetic moments are aligned) even in the absence of an external magnetic field.
This indicates that there is a strong internal field within the material which makes the atomic magnetic moments align with each other.
This phenomenon is known as ferromagnetism
EXCHANGE INTERACTION AND FERROMAGNETISM
The
ferromagnetic property is exhibited by transition elements such as iron,
cobalt, and nickel at room temperature and rare earth elements like gadolinium
and dysprosium.
The
ferromagnetic materials possess parallel alignment of dipoles. This parallel
alignment of dipoles is not due to the magnetic force existing between any two
dipoles. The reason is that the magnetic potential energy is very small and it
is smaller than thermal energy.
The
electronic configuration of iron is 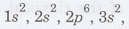
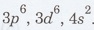 . For iron, the 3d subshell
is an unfilled one. This 3d subshell have five orbitals.
. For iron, the 3d subshell
is an unfilled one. This 3d subshell have five orbitals.
For
iron, the six electrons present in the 3d subshell occupy the orbitals such
that there are four unpaired electrons and two paired electrons as shown in
figure 2.28.
These
four unpaired electrons contribute a magnetic moment of 4ẞ. This arrangement
shows the parallel alignment of four unpaired electrons.

The
parallel alignment of dipoles in iron is not due to the magnetic interaction.
It is due to the Pauli's exclusion principle and electrostatic interaction
energy.
The
Pauli's exclusion principle and electrostatic are combined together and
constitute a new kind of interaction
known as exchange interaction. The exchange interaction is a a quantum
mechanical concept.
The exchange interaction between any two atoms depends upon the interatomic separation between the two interacting atoms and the relative spins of the two outer electrons. The exchange interaction between any two atoms is given by
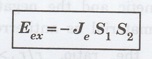
where
Je is the numerical value of the exchange integral, S1
and S2 are the spin angular momenta of the first and second
electrons.
The
exchage integral value is negative for a number of elements. Therefore, the
exchange energy value is negative (minimum energy energy configuration) when
the spin angular momentum S1 and S2 are opposite
direction.
Hence,
antiparallel alignment of dipole is favoured. This explains the antiparallel
ben alignment of dipoles in antiferromagnetic materials.
In
some materials like iron, cobalt and nickel the exchange integral value is
positive. The exchange energy is negative when the spin angular momentum is in
the same direction. This will produce a parallel alignment of dipoles.
A
plot between the exchange integral and the ratio of the interatomic separation
to the radius of 3d orbital (r/rd) is shown in figure
2.29.
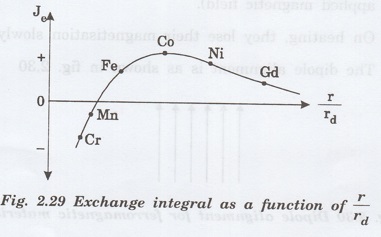
For
the transition metals like iron, cobalt, nickel and gadolinium the exchange
integral is positive, whereas for manganese and chromium the exchange integral
is negative.
The
positive value of the exchange integral represents the material as
ferromagnetic and the negative exchange integral value represents the material
as antiferromagnetic.
In
general, if the ratio, r/rd >3, the material is ferromagnetic,
otherwise the material is antiferromagnetic. It should be noted that manganese
is suitably alloyed so that r/rd >3, and it will become
ferromagnetic.
Ferromagnetic
materials
The
materials which exhibit the ferromagnetism are 18lug called ferromagnetic
materials.
Properties
i.
All the dipoles are aligned parallel to each other due to the magnetic
interaction between the dipoles.
ii.
They have ey have permanent dipole pole moment. They are strongly attracted by
the magnetic field.
iii.
They exhibit magnetisation even in the absence of magnetic field. This property
of ferromagnetic materials is called as spontaneous magnetisation.
iv.
They exhibit hysteresis (lagging of magnetisation with applied magnetic field).
v.
On heating, they lose their magnetisation slowly.
vi.
The dipole alignment is as shown in fig. 2.30

Fig. 2.30 Dipole alignment for ferromagnetic materials
vii.
The magnetic susceptibility is very high and it depends on temperature.
It
is given by

where
C is Curie constant and 0 ferromagnetic Curie temperature.
Physics for Electronics Engineering: Unit II: Electrical and Magnetic Properties of Materials : Tag: : Exchange Interaction and Ferromagnetism - Ferromagnetic Effect
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
