Physics for Electronics Engineering: Unit II: Electrical and Magnetic Properties of Materials
Electrical Properties of Materials
It is essential to study the various electrical properties of the solids for their specific applications.
Introduction
It
is essential to study the various electrical properties of the solids for their
specific applications.
In
terms of electrical properties, all solid state materials are classified into
three groups as conductors, semiconductors and dielectrics or insulators.
The
selection of materials for different applications depends on their electrical
properties as well as requirements of the job.
Electrical
phenomena are caused by the motion of electrons in solids and find many
applications in day-to-day activities.
Electrons
in a metallic filament and the property of electrical resisivity of the
material are used in incandescent lamps for heating and illumination in a
variety of domestic and industrial applications.
In
recent times, the mobility of electrons is being exploited in solar cells,
lasers, in the control of thermonuclear reactions and many other applications.
The chapter mainly deals with the electrical conduction, thermal conduction, density of states, number of electrons per unit volume in a metal etc.
Besides,
it also deals with the classical, quantum free electron theories and band
theory to explain the conductivity of the solid state materials.
Basic definition
It
is necessary to know the basic relations in electrical conductivity to
understand its derivation.
Ohm's Law
When
an electrical current flows through a conductor, then the voltage drop across
the conductor is given by the ohm's law
V
= IR (or) I = V/R .................(1)
where
I - current in ampere,
R
- resistance to the current flow in ohm
V
- voltage drop across the conductor in volt.
Resistance (R)
The
resistance R of a conductor is a geometry (length and area) and property
dependent factor of the material used.
R
α l / A
R
= p l / A = l / σ A .................(2)
p -
Proportionality constant known as resistivity (ohm m) [p = 1/σ]
l - Length of the
conductor (m)
A
- Area of cross section (m2)
σ - Electrical conductivity (ohm-1 m-1) (or) (mho m-1) (or) siemens m-1 (Sm-1)
Current Density (J)
It
is defined as the current per unit area of cross section of a current carrying
conductor. If I is the current and A is the area of cross-section, then current
density is given by
J
= I/A .................(3)
Its unit is Am-2
Electrical Field (E)
The
electrical field E in a conductor of uniform cross section is defined as the
potential drop (voltage) V per unit length.
E
= V / l .................(4)
Its unit is Vm-1
Electrical Conductivity (σ)
The
amount of electrical charges (Q) conducted per unit time across unit area (A)
of a conductor per unit applied electrical field (E) is (E) is defined as
electrical conductivity.
It
is denoted by σ and it is given by
σ
= Q / t A E
σ
= Q / t A E = J / E
where
J is the current density and it is given by (Q / t A)
A second form of ohm's law is obtained by combining equations (1) and (2)
From
eqn (1), V = IR
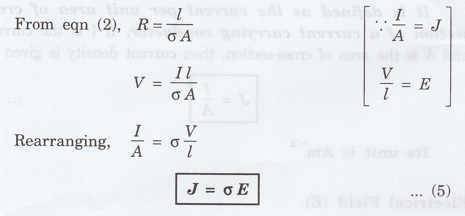
Relation between Current Density J,
Drift Velocity vd and
Mobility μ
Let
n be the number of charge carriers per unit volume (also called charge carrier
density) in a conductor of length with uniform cross sectional area A. The
current flow through the conductor is given by
I
= Total charge / Time = Q / t
=
n e Al / t = n e A vd .................(6)
where
vd = l / t is called the drift velocity. It is the average velocity
gained by the charge carriers in the presence of an electrical field.
But,
we know that J = I / A
Using
the eqn (6), J is written as
J
= n e A vd / A = n e vd
J = n e vd .................(7)
But
J = σ E.
Therefore,
the eqn (7) becomes
σ
E = n e vd
σ
= n e vd / E
Hence,
σ = n e μ .................(8)
where
μ = vd / E is called the
mobility of the charge carrier. It is defined as the drift velocity per unit
electric field. Its unit is m2 V-1s-1.
Free Electron theory of solids
It
is well known that the electrons in the outermost orbit of the atom determine
the electrical properties of a solid. The free electron theory of solids
explains the structure and properties of solids through their electronic
structures.
This
theory is applicable to all solids, both metals and non-metals. It explains
i.
The behaviour of conductors, semiconductors and insulators.
ii.
The electrical, thermal and magnetic properties of solids.
Main Stages of Free Electron theory
of solids
(i) Classical free electron theory (Drude
and Lorentz free electron theory)
This
theory was proposed by Drude and Lorentz in the year 1900. According to this
theory, the free electrons are mainly responsible for electrical conduction in
a metal.
It obeys the laws of classical mechanics. Here, the free electrons are assumed to move in a constant potential.
(ii) Quantum free electron theory (Sommerfeld
Quantum theory)
Quantum
free electron theory was proposed by Sommerfeld in the year 1928. According to
this theory, the electrons in a metal move in a constant potential.
It
obeys the laws of quantum mechanics. The wave nature of electron is taken into
account to describe the electron.
(iii) Zone theory or band theory of
solids
This
theory was proposed by Bloch in the year 1928. According to this theory, free
electrons move in a periodic potential.
Physics for Electronics Engineering: Unit II: Electrical and Magnetic Properties of Materials : Tag: : - Electrical Properties of Materials
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
