Physics for Electronics Engineering: Unit I: Crystallography
Zinc Sulphide (ZnS) Structure
Crystallography
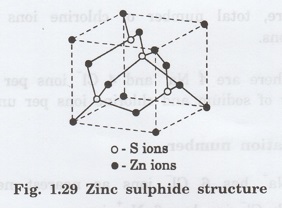
Zinc sulphide structure is also known as the Zinc blende structure. This structure is identical to diamond structure except that two interpenetrating FCC sub- lattices are of different atoms one composed entirely of zinc atoms and the other entirely of sulphur atoms.
ZINC
SULPHIDE (ZnS) STRUCTURE
Zinc sulphide structure is also known as
the Zinc blende structure. This structure is identical to diamond structure
except that two interpenetrating FCC sub- lattices are of different atoms one
composed entirely of zinc atoms and the other entirely of sulphur atoms. (fig.
1.29).
This is therefore true face-centered
cubic structure with a basis of two different atoms.
Formation
of structure
We know the diamond structure is made by
carbon atoms [corner + face centred +4 atoms inside the unit cell].
In the diamond structure if the corner
and face centred atoms are replaced by sulphur (S-) atoms and four
atoms present inside the unit cell are replaced by the zinc (Zn+)
atoms, then we get zinc sulphide structure. It is shown as in fig. 1.29. It
also has the coordination number as 4.
Example
Many important compounds such as AlAs,
GaAs, GaP, GaSb, InAs, InP, InSb, ZnS, ZnSe, Cds, CuCl etc. have this type of
structure.
Density
of Crystal
This is defined as:
Density of the crystal (p) = Mass of
unit cell / Volume of unit cell
Mass of unit cell = (Atomic mass /
Avogadro number) × Number of atoms per unit cell
= (M/NA) x n
n → number of atoms per unit cell and
NA → Avogadro's number,
M → the atomic weight and
a → side of a cubic unit cell
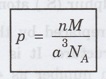
Physics for Electronics Engineering: Unit I: Crystallography : Tag: : Crystallography - Zinc Sulphide (ZnS) Structure
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
