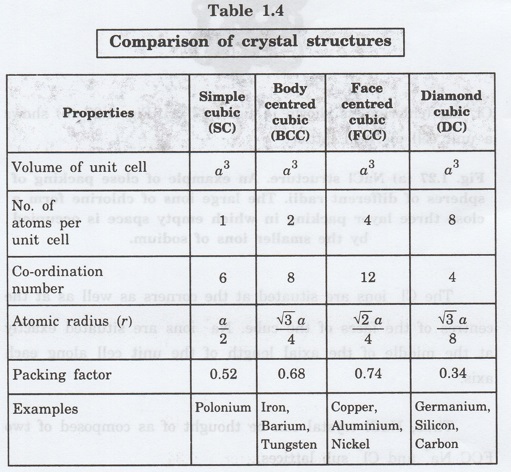
Physics for Electronics Engineering: Unit I: Crystallography
Diamond Cubic (DC) Structure
The diamond cubic structure is a very important crystal structure. Besides diamond, the elemental semiconductors silicon and germanium also have this structure.
DIAMOND
CUBIC (DC) STRUCTURE
The diamond cubic structure is a very
important crystal structure. Besides diamond, the elemental semiconductors
silicon and germanium also have this structure. The unit cell of diamond cubic
structure is shown in fig. 1.24.
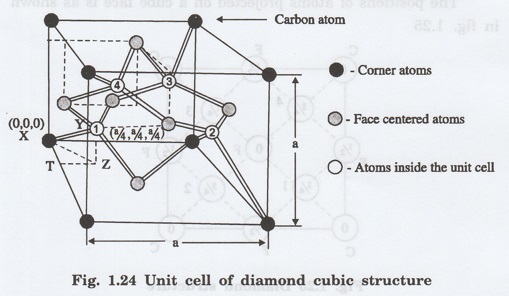
This structure is a combination of two
interpenetrating face-centred - cubic (FCC) sub lattices.
One sub- lattice has its origin at (0,
0, 0) (atom X). The other sub- lattice has its origin (atom Y) quarter of the
way along the body diagonal ie., at the point (a/4, a/4, a/4)
It is loosely packed structure since
each atom has only four nearest neighbours (ie., coordination number is 4).
1. Number of atoms per unit cell
In the unit cell of diamond, the carbon
atoms are present at three different positions of the unit cell as shown in
fig. 1.24.
(i) Corner atoms represented by 'C'.
(ii) Face centred atoms represented by
'F'.
(iii) Four atoms present fully fully
represented as 1, 2, 3 and 4.
The positions of atoms projected on a
cube face is as shown in fig. 1.25
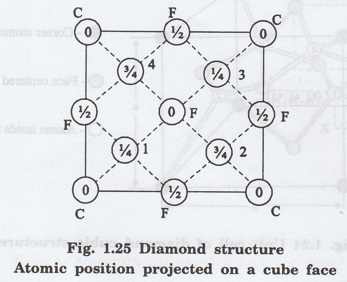
(i)
Number of corner atoms per unit cell
Each corner atom is shared by 8 unit
cells. There are 8 corner atoms in the unit cell.
Therefore, the number of atoms due to
corner atoms per unit cell = (1/8) x 8 = 1 atom
(ii)
Number of face centred atoms per unit cell
Each face centred atom is shared by 2
unit cells. We have 6 face centred atoms.
Number of face centred atom per unit
cell = (1/2) x 6 = 3 atoms
(iii)
Number of atoms inside unit cell
Inside the unit cell, we have 4 atoms
represented by 1, 2, 3, 4 in fig. 1.26 which are not shared by any other
sourrounding unit cells.
Total number of atoms per unit cell = 1 +
3 + 4 = 8
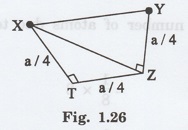
2. Atomic radius
The corner atoms do not touch each
other. Similarly the face centred atoms also do not touch each other.
But both face centred atoms and corner
atoms touch with the atoms (1, 2, 3, 4) situated inside the unit cell as shown
in fig. 1.26.
For example, the nearest two neighbours
which have direct contact (shown by double line) are atoms 'X' and 'Y as shown
in fig 1.26.
A perpendicular is drawn to Y atom which
meets the unit cell at a point 'Z' as shown in fig. 1.26 which is at a distance
own in fig. 1.26 which is at a distance of a/4.
XY2 = XZ2 + ZY2
XY2 = XT2 + TZ2
+ ZY2 [XZ2 = XT2 + TZ2]
Substituting for XT, TZ and ZY, we have
XY2 = (a/4)2 +
(a/4)2 + (a/4)2
XY2 = a2/42
+ a2/42 + a2/42
XY2 = a2/16 + a2/16
+ a2/16
XY2 = 3a2/16
Taking square root on both sides, we
have
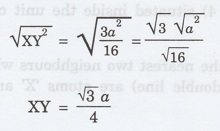
Since XY = 2r, we have
2r = √3 a/4
r = (√3 a)/(4 ×2)
r = √3 a/8
Atomic radius r = √3 a/8
3. Coordination number
From fig. 1.26 the number of nearest
atoms (shown by double line) for Y atom is 4. Therefore, the coordination
number for diamond is 4.
Note:
The coordination number is found to be same even if it is calculated with
respect to atoms say (2), (3), (4), corner (or) face centred atoms.
4. Packing factor
We know that Packing factor (PF)
= Volume occupied by the atoms per unit
cell (v) / Volume of the unit cell (V) .................(1)
Volume occupied by 1 atom = (4/3) πr3
In diamond, we have 8 atoms per unit
cell
Volume occupied by all the '8' atoms per
unit cell (v) = 8 x (4/3) πr3
We know that atomic radius for diamond
structure
r = √3 a / 8
Volume occupied by the atoms per unit
cell
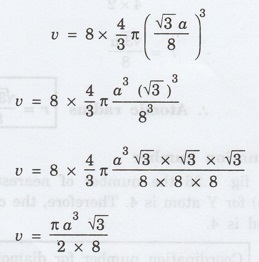
Volume occupied by the atoms per unit
cell

Since diamond has cubic structure, the
volume of the unit cell
V =
Substituting the equations (2) and (3)
in (1) we get

Packing factor = 0.34 = 0.34 x 100 %
Packing factor = 34%
Thus only 34% volume of the unit cell in
diamond cubic structure is occupied by atoms and the remaining 66% volume is
vacant.
Since the packing density is very low,
it is termed as very loosely packed structure.
Note:
Packing factor of diamond cubic = 1/2 of packing factor of BCC
Physics for Electronics Engineering: Unit I: Crystallography : Tag: : - Diamond Cubic (DC) Structure
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
