Physics for Electronics Engineering: Unit I: Crystallography
Sodium Chloride Structure
Crystallography
Ionic solids are made up of cation (+ ion) and anions (- ion). Generally, the cations (+ ve) are smaller in size to anions. Sodium chloride and many other ionic crystals crystallize in rock salt structure which is also known as sodium chloride structure.
SODIUM
CHLORIDE STRUCTURE
Ionic solids are made up of cation (+
ion) and anions (- ion). Generally, the cations (+ ve) are smaller in size to
anions.
Sodium chloride and many other ionic
crystals crystallize in rock salt structure which is also known as sodium
chloride structure.
Structure composition
NaCl crystal has FCC structure with Na+
ion (sodium) and
Cl- ion (chlorine) is shown
in fig. 1.27 (a). Fig. 1.27 (b) shows a unit cell of NaCl lattice.
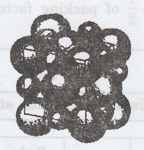
Fig. 1.27 (a) NaCl structure. An example
of close packing of spheres of different radii. The large ions of chlorine form
a close three layer packing in which empty space is occupied by the smaller
ions of sodium.
The Cl- ions are situated at
the corners as well as at the centres of the faces of the cube. Na+
ions are situated exactly at the middle of the axial length of the unit cell
along each axis.
Thus, NaCl crystal can be thought of as
composed of two FCC Na+ and
Cl- sub lattices.
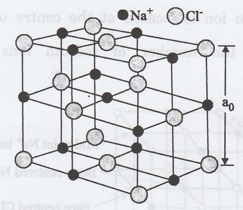
Fig. 1.27 (b) Model of unit cell of NaCl structure
When one of Cl- ions has the origin at (0, 0, 0) then one of the nearest Na+ ion has the origin at
(1/2, 0, 0)
Let us discuss the important parameters
of the NaCl crystal.
1. Number of atoms per unit cell
In NaCl structure, it has two types of
ions namely, Na+ and Cl-. Let us find the number of
sodium ions and chlorine ions separately.
(a)
Number of Na+ ions per unit cell
Na+ ion is located at the mid
point of the axial length. There are '12' such mid point Na+ ions.
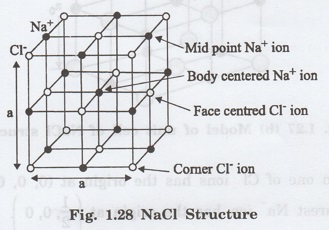
Each sodium ion is shared by 4 adjacent unit cells. (Fig. 1.28)
Share of one unit cell ie.,
number of mid point Na+ ions
per unit cell = (1/4) × 12 = 3 ions
One sodium ion is located at the centre of the unit cell.
Therefore, the number of sodium ions per
unit cell = 3+1 = 4 ions.
(b)
Number of CI ions per unit cell
Here, there are two types of Cl-
ion namely (a) corner Cl- ions and (b) face centered Cl- ions
as shown in fig. 1.28
There are 8 chlorine ions in the corners
and they are shared by 8 adjacent unit cells.
Number of corner Cl- ions per
unit cell = (1/8) x 8 = 1 ion
Each face centered Cl- ion is
shared by 2 adjoining unit cell. There are 6 face centered Cl- ions.
Number of face centered Cl-
ion per unit cell = (1/2) x 6 = 3 ions
Therefore, total number of chlorine ions per unit cell = 1+3 = 4 ions.
Thus, there are 4 Na+ and 4 Cl-
ions per unit cell. i.e., total number of sodium and chlorine ions per unit
cell is 8.
2. Co-ordination number
Each Na+ has 6 Cl-
ions as nearest neighbours and similarly each Cl- ion has 6 Na+
ions as nearest neighbours. Hence, the coordination number of NaCl for opposite
kind of ions is 6.
3. Nearest neighbouring distance
Nearest neighbouring distance is a/2
Physics for Electronics Engineering: Unit I: Crystallography : Tag: : Crystallography - Sodium Chloride Structure
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
