Physics for Electronics Engineering: Unit I: Crystallography
Crystal Systems
7 types with Example
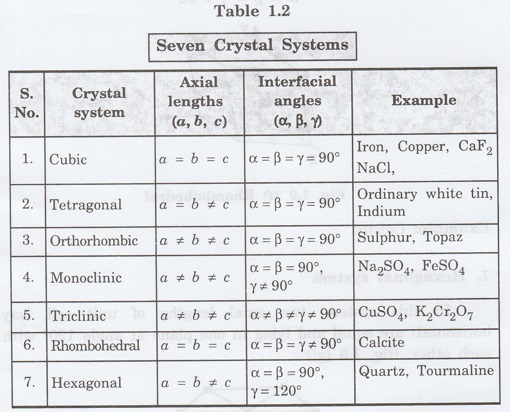
There are '7' types of crystal systems. They are 1. Cubic, 2. Tetragonal, 3. Orthorhombic, 4. Monoclinic, 5. Triclinic, 6. Rhombohedral, 7. Hexagonal
CRYSTAL
SYSTEMS
There are '7' types of crystal systems.
They are
1. Cubic
2. Tetragonal
3. Orthorhombic
4. Monoclinic
5. Triclinic
6. Rhombohedral
7. Hexagonal
1. Cubic system
In this crystal system, all the three
axial lengths of the unit cell are equal and they are perpendicular to each
other (fig. 1.9 [a]).
i.e., a = b = c and
α = β = γ = 90°
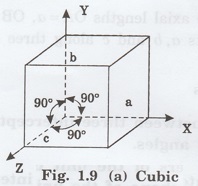
Example: Iron, Copper, Sodium Chloride
(NaCl), Calcium Fluoride (CaF2).
2. Tetragonal system
In this system, two axial lengths of the
unit cell are equal and third axial length is either longer or shorter (fig.
1.9 [b]). All the three axes are perpendicular to each other.
i.e., a = b ≠ c and
α = β = γ = 90°
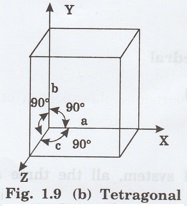
Example: White tin, Indium.
3. Orthorhombic system
In this system, three axial lengths of
the unit cell are not equal but they are perpendicular to each other. (fig. 1.9
[c]).
i.e., a ≠ b ≠ c and
α = β = γ = 90°
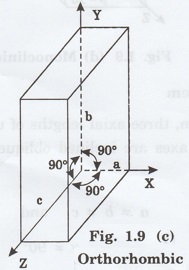
Example: Sulphur, Topaz.
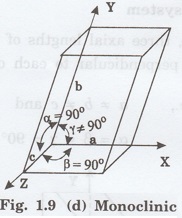
4. Monoclinic system
In this system, three axial lengths of
unit cell are not equal. Two axes are perpendicular to each other and third
axis is obliquely inclined (fig. 1.9 [d]).
i.e., a ≠ b ≠ c and
α = β = 90°, γ ≠ 90°
Example: Sodium sulphite (Na2
SO3), Ferrous sulphate (FeSO4).
5. Triclinic system
In this system, three axial lengths of
unit cell are not equal and all the three axes are inclined obliquely to each
other (fig. 1.9 [e]).
i.e., a ≠ b ≠ c and
α ≠
β ≠ γ ≠ 90°
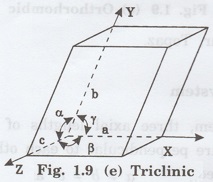
Example: Copper sulphate (CuSO4),
Potassium dichromate (K2Cr2O7)
6. Rhombohedral system (Trigonal)
In this system, three axial lengths of
the unit cell are equal. They are equally inclined to each other at an angle
other than 90° (fig. 1.9 [f]).
i.e., a = b = c and
α = β = γ ≠ 90°
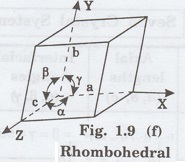
Example: Calcite.
7. Hexagonal system
In of unit cell (say this system, two
axial lengths horizontal) are equal and lying in one plane at angle 120° with
each other. (fig. 1.9 [g]).
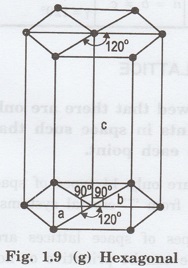
The third axial length (say vertical) is
either longer or shorter than other two and it is perpendicular to this plane.
i.e., a = b ≠ c and
α = β = 90°, γ = 120°
Example: Quartz, Tourmaline.
Physics for Electronics Engineering: Unit I: Crystallography : Tag: : 7 types with Example - Crystal Systems
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
