Physics for Electronics Engineering: Unit I: Crystallography
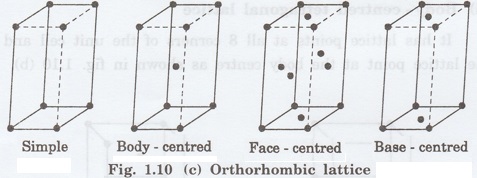
Bravais Lattice
Crystallography
Bravais showed that there are only 14 possible ways of arranging points in space such that the environment looks same from each point.
BRAVAIS
LATTICE
Bravais showed that there are only 14
possible ways of arranging points in space such that the environment looks same
from each point.
Thus, there are only 14 types of space
lattices which are possibly developed from '7' crystal systems.
These 14 types of space lattices are
known as Bravais lattices. The lattice can be primitive or non-primitive.
Primitive
cell
A primitive cell is the simplest type of
unit cell which contains only one lattice point per unit cell (contains lattice
it cell (contains lattic points only at the corners of unit cell).
Example:
Simple Cubic (SC), Simple Tetragonal
Non
- primitive cell
The unit cell which contains more than
one is called non- primitive cell.
Example:
BCC, FCC and HCP contains more than one lattice point per unit cell.
If the number of lattice points per unit
cell is two (BCC), three and four (FCC), then the unit cell is called doubly
primitive, triply primitive and quadruply primitive respectively.
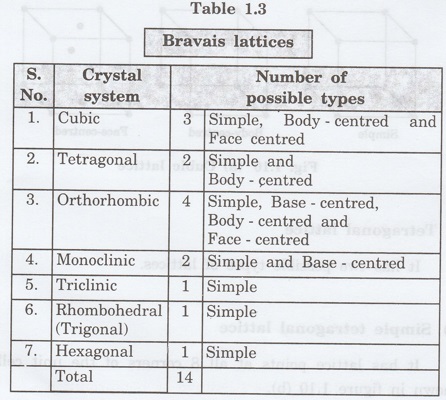
1. Cubic lattice
It has 3 possible types of arrangements
of lattice points.
(i)
Simple (or primitive) cubic lattice (SC)
It has lattice points at all 8 corners
of the unit cell as shown in fig. 1.10 (a).
(ii)
Body-centred cubic (bcc) lattice
It has lattice points at all 8 corners
of the unit cell and one lattice point at the body centre as shown in fig. 1.10
(a).
(iii)
Face - centred cubic (fcc) lattice
It has lattice points at all 8 corners
of the unit cell and one lattice point at each face centre of 6 faces of the
cube as shown in fig. 1.10 (a).
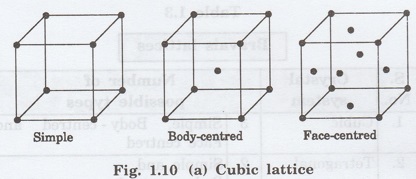
2. Tetragonal lattice
It has two possible types of lattices.
(i)
Simple tetragonal lattice
It has lattice points at all 8 corners
of the unit cell as shown in figure 1.10 (b).
(ii)
Body-centred tetragonal lattice
It has lattice points at all 8 corners
of the unit cell and one lattice point at the body centre as shown in fig. 1.10
(b).
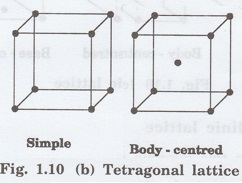
3. Orthorhombic lattice
It has four possible types of lattices.
(i)
Simple orthorhombic lattice
It has lattice points at all 8 corners
of the unit cell as shown in fig. 1.10 (c).
(ii)
Body centred orthorhombic lattice
It has lattice points at all 8 corners
of the unit cell and one lattice point at the body centre as shown in fig. 1.10
(c).
(iii)
Face - centred orthorhombic lattice
It has lattice points at all 8 corners
of the unit cell and one lattice point at each face centre of the 6 faces of
the unit cell as shown in fig. 1.10 (c).
(iv)
Base- centred orthorhombic lattice
It has lattice points at all 8 corners
of the unit cell and 2 lattice points each at the centre of two faces (base)
opposite to each other as shown in fig. 1.10 (c).
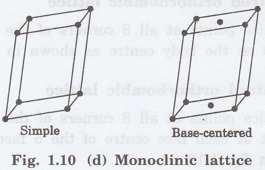
4. Monoclinic lattice
It has two possible space lattices.
(i)
Simple monoclinic lattice
It has lattice points at all 8 corners
of the unit cell as shown in fig. 1.10 (d)
(ii)
Base - centred monoclinic lattice
It has lattice points at all 8 corners
of the unit cell and 2 lattice points each at the centre of two faces (faces of
the base) opposite to each other as shown in fig. 1.10 (d)
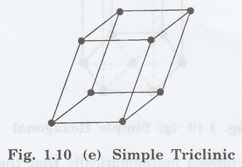
5. Triclinic lattice
It has only one possible space lattice.
Simple
Triclinic lattice
It has lattice points at all 8 corners
of the unit cell as shown in fig. 1.10 (e).
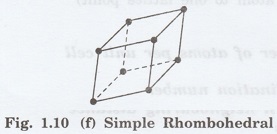
6. Rhombohedral lattice
It has only one possible space lattice.
Simple
Rhombohedral lattice
It has lattice points at all 8 corners
of the unit cell as shown in fig. 1.10 (f)
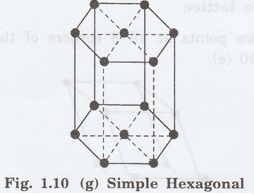
7. Hexagonal lattice
It has only one possible space lattice.
Simple
Hexagonal lattice
It has lattice points at all 12 corners
of the hexagonal unit cell and 2 lattice points each at the centre of two
hexagonal faces of the unit cell (top and bottom) as shown in fig. 1.10 (g).
In fact, it is proved mathematically
that there are only 14 independent ways of arranging points in three
dimensional space such that each arrangement confirms to the definition of
space lattice.
Characteristics of the unit cell
The unit cell is characterized by the
following parameters: (Assuming one atom to one lattice point)
(i) Number of atoms per unit cell
(ii) Coordination number
(iii) Nearest neighbouring distance
(iv) Atomic radius
(v) Packing factor
(i) Number of atoms per unit cell
It is the number of atoms possessed by
the unit cell. This is determined by the arrangement of atoms in the unit cell
(ii) Coordination Number (CN)
It is the number of nearest atoms
directly surrounding a particular atom in a crystal.
The coordination number gives the
information about the packing of atoms in a structure. It tells whether the
crystal structure is closely packed or loosely packed.
If the coordination number is high, then
the structure is more closely packed. If it is low, then the structure is
loosely packed.
(iii) Nearest neighbouring distance
(2r)
It is the distance between the centres
of two nearest neighbouring atoms.
It is expressed in terms of the length
of edge of the unit cell 'a' and it is 2r in simple cubic. (Fig. 1.11)
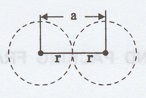
Fig. 1.11 Nearest neighbouring distances
atomic radius
(iv) Atomic radius (r)
It is half of the distance between two
nearest neighbouring atoms in a crystal. It is denoted by 'r'. It is usually
expressed in terms of cube edge 'a' (lattice parameter).
For a simple cubic unit cell, the atomic
radius. (Fig. 1.11).
r = a/2
(v) Packing Factor (PF)
It is defined as the ratio of total
volume occupied by the atoms in a unit cell to the total volume of a unit cell.
Packing factor = Total volume occupied
by the atoms in a unit cell (v) / Total volume of the unit cell (V)
= v / V
= Number of atoms per unit cell × Volume
of one atom / Total volume of the unit cell
It is also known as density of packing.
The packing factor tells us how closely
the atoms are stacked in the unit cell. A high packing factor indicates that
atoms are very closely packed and therefore there is very little unoccupied
space.
On the other hand, a low packing factor
indicates loose packing of atoms and hence there is relatively more unoccupied
space.
Physics for Electronics Engineering: Unit I: Crystallography : Tag: : Crystallography - Bravais Lattice
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
