Physics for Electronics Engineering: Unit I: Crystallography
Body - Centred Cubic (BCC) Structure
In this crystal structure, the unit cell has one atom at each corner of the cube and one atom at the body centre of the cube
BODY
- CENTRED CUBIC (BCC) STRUCTURE
In this crystal structure, the unit cell
has one atom at each corner of the cube and one atom at the body centre of the
cube 1.16 (a). The atoms represented as hard spheres are shown in fig. 1.16
(b).

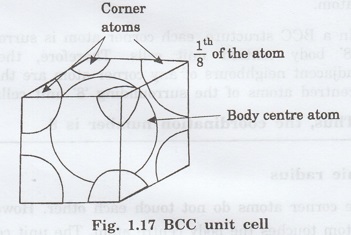
1. Number of atoms per unit cell
The unit cell of the BCC structure is
shown in fig. 1.17.
Each corner atom is shared by 8
surrounding unit cells.
Hence, the share of one unit cell =
(1/8) of corner atoms.
There are '8' corner atoms,
Contribution of all corner atoms = (1/8)
× 8 = 1 atom
There is one atom at the body centre of
each unit cell.
Total number of atoms in one unit cell =
1 + 1 = 2 atoms
2. Coordination number
In the unit cell of BCC structure, there
is one atom (say atom X) at the body centre of the unit cell. There are '8'
atoms at the 8 corners of the unit cell as shown in fig. 1.18.
The corner atoms do not touch each
other. But all the eight corner atoms touch the body centre atom along the body
diagonal. Thus, for body centre atom 'X', there are 8 nearest neighbours (ie.,
8 corner atoms).
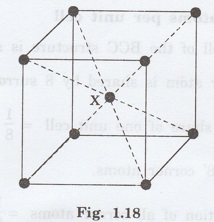
Hence, the coordination number of body
centred cubic structure is 8.
Note:
The coordination number can also be determined in another way by taking the
corner atom as reference atom.
In a BCC structure, each corner atom is
surrounded by '8' body centred unit cells. Therefore, the nearest adjacent
neighbours of any corner atom are the '8' body centred atoms of the surrounding
'8' unit cells.
Thus, the coordination number is 8.
3. Atomic radius
The corner atoms do not touch each
other. However, each corner atom touches the body centre atom. The unit cell of
BCC is shown in fig. 1.19. The side of the unit cell is 'a'.
Consider the atoms at A, G and at the
centre of the unit cell 'O'.
It is clear from fig. 1.19 that the
corner atoms A and G are nearest neighbouring atoms to the body centre atom O.
These atoms lie in a straight line along
the body diagonal AG of the cube.
From the geometry of figure 1.19,
AG = r + 2r + r = 4r
on squaring on both sides, we get
AG2 = (4r)2
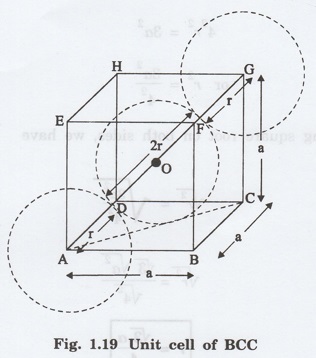
From the right angled ∆ ABC,
AC2 = AB2 + BC2
substituting for AB and BC from the fig
1.19, we have
AC2 = a2 + a2
AC2 = 2a2
From the right angled ∆ ACG,
AG2 = AC2 + CG2
Substituting for AG2, AC2
and CG2, we have
(4r)2 = 2a2 + a2
= 3a2
42 r2 = 3a2
Or r2 = 3a2/4
Taking square root on both sides, we
have
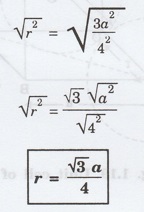
4. Packing factor
Number of atoms per unit cell = 2
Volume of 2 atoms in the unit cell, v =
2 x (4/3)πг3
Atomic radius r = √3 a/4
Volume of the unit cell, V = a3
Packing factor = v/V
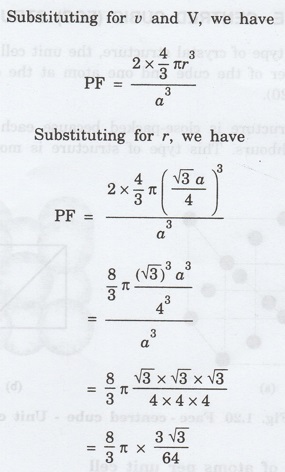
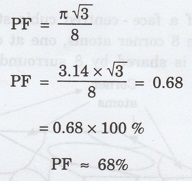
Thus, packing factor is 68% ie., 68% of
the volume of unit cell is occupied by atoms and the remaining 32% volume is
vacant.
Common
examples of this type of structure
Tungsten, Chromium and Molybdenum.
Physics for Electronics Engineering: Unit I: Crystallography : Tag: : - Body - Centred Cubic (BCC) Structure
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
