Physics for Electronics Engineering: Unit I: Crystallography
Basis of Crystal structure
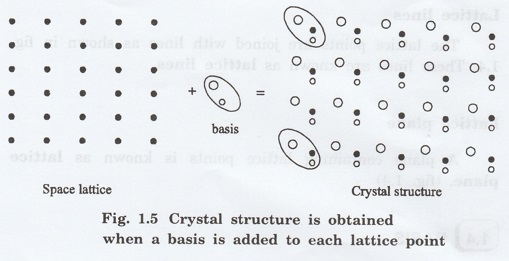
The crystal structure is obtained by adding a unit assembly of atoms to each lattice point. This unit assembly is called basis.
BASIS
Definition
The crystal structure is obtained by
adding a unit assembly of atoms to each lattice point. This unit assembly is
called basis.
Explanation
A basis may be a single atom or assembly
of atoms which is identical in composition, arrangement and orientation.
When the basis is repeated in a space
lattice with correct periodicity in all three directions, then it gives the
actual crystal structure.
Therefore, a space lattice combined with
a basis gives a crystal structure.
ie., Space lattice + Basis → Crystal
structure
The basis combined with lattice points
is shown in fig 1.5 in which two atoms (represented by circles of smaller and
large radii) are added to one lattice point (represented by a black dot).
For many metals, the number of atoms in
basis is one (Aluminium and Barium crystals), two or three or more.
For example in NaCl and KCl, each basis
has two atoms and in CaF2, basis has three atoms. But, for many
complicated structures, the basis exceeds more than 1000 atoms.
Note
A space lattice refers to the geometry
of a set of points in space whereas a crystal structure refers to the actual
arrangement of atoms in space.
Physics for Electronics Engineering: Unit I: Crystallography : Tag: : - Basis of Crystal structure
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
