Physics for Electronics Engineering: Unit I: Crystallography
Anna University Solved Problems
Crystallography | Physics for Electronics Engineering
Anna University solved problems in Crystallography
ANNA
UNIVERSITY SOLVED PROBLEMS
Problem
1.6
Lattice constant of a BCC crystal is
0.36 nm. Find its atomic radius. [A.U. Jan 2013]
Given
data
a = 0.36 nm = 0.36 x 10-9 m
Solution
For BCC, r = a√3 / 4
Substituting the given value, we have

Problem
1.7
Copper is FCC whose atomic radius is
1.26 x 10-10 m. Calculate its lattice constant. (A.U. May 2012)
Given
data
r = 1.26 x 10-10 m.
Solution
For FCC, r = a √2 / 4
a = 4r / √2
substituting the given value, we have
a = (4 x 1.26 x 10-10) / √2 m
a = 3.56 x 10-10 m
a = 3.56 Å
Problem
1.8
Copper has a fcc structure and its
atomic radius is 1.273 Å. Find (1) lattice parameter and (2) density of copper.
Atomic weight of copper = 63.5
Avagadro's number = 6.02 × 1026
mol–1 (A.U. Jan 2012)
Given
data
Atomic radius for fcc system r = 1.273 Å
= 1.273 x 10-10 m
Atomic weight of copper M = 63.5
Avagadro's number N = 6.02 x 1026
mol-1
Number of atoms per unit cell n = 4
Solution
Lattice parameter a = 4r / √2
a = (4 x 1.273 x 10-10) /
1.414
Lattice constant = 3.60 × 10-10
m
We know that density p = nM / Na3
Substituting the given values, we have
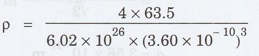
Density of copper p = 9043 kg m-3
Problem
1.9
α - iron of atomic weight 55.85
solidifies into BCC structure and has a density 7860 kg m-3.
Calculate the radius of an atom. (A.U.
Dec 2012)
Given
data
Atomic weight M = 55.85
Density p = 7860 kg m-3
Number of atoms per unit cell for BCC =
2
Avagadro's number N = 6.023 x 1026
mol-1
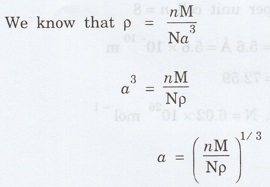
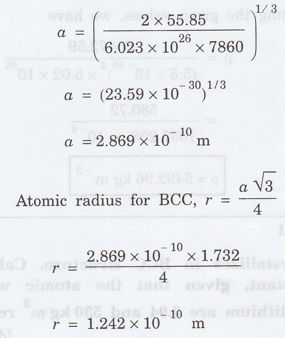
Problem
1.10
Ge crystallizes in diamond (form)
structure with 8 atoms per unit cell. If the lattice constant is 5.6 Å
Calculate its density. (A.U. May 2012)
Given data
Number of atoms per unit cell n = 8
Lattice constant a = 5.6 Å = 5.6 × 10-10
m
Atomic weight, M = 72.59
Avagadro's number, N = 6.02 x 1026
mol-1
Solution
We know that p = nM / a3N
Substituting the given values, we have

Problem
1.11
Lithium crystallizes in BCC structure.
Calculate the lattice constant, given that the atomic weight and density for
lithium are 6.94 and 530 kg m3 respectively. (AU. Jan 2011)
Given
Data
Atomic weight M = 6.94
Density p =530 kg m-3
No. of atoms per unit cell for BCC, n =
2
Avagadro's number = 6.02 x 1026
kg mol-1
Solution
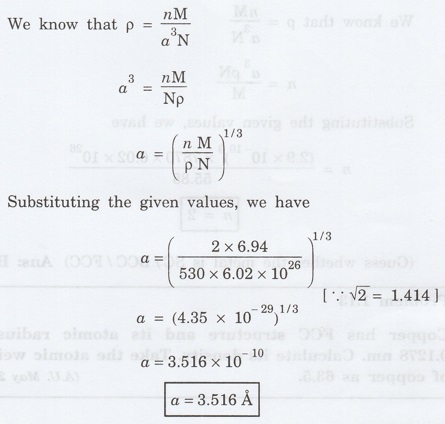
Problem
1.12
Calculate the number of atoms per unit
cell of a metal with lattice parameter 2.9 Å. Given molecular weight 55.85,
density 7870 kg m-3 and Avagadro's number 6.02 x 1026 mol-1
(A.U. May 2012)
Given
data
Lattice parameter a = 2.9 Å = 2.9 x 10-10
Molecular weight = 55.85
Density p = 7870 kg m-3
Avagadro's number, N = 6.02 x 1026
mol-1
Solution
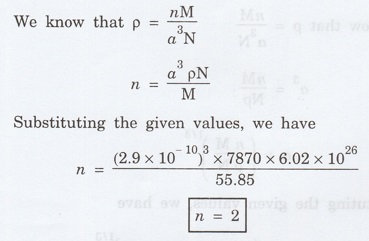
(Guess whether the metal is SC/BCC/FCC)
Ans: BCC
Problem
1.13
Copper has FCC structure and its atomic
radius is 0.1278 nm. Calculate its density. Take the atomic weight of copper as
63.5. (A.U. May 2011)
Given
data
Atomic radius r = 0.1278 nm = 0.1278 ×
10-9 m
Atomic weight = 63.5
Avagadro's number N = 6.02 × 1026
mol-1
Number of atoms per unit cell for FCC, n
= 4
Solution
We know that atomic radius for FCC
r = √2 a / 4
a = 4r / √2
substituting the given values, we have
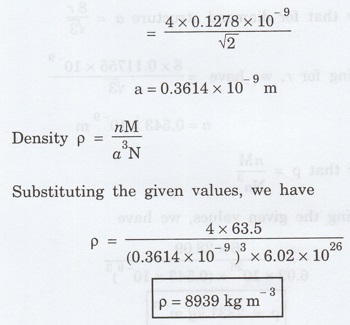
Problem
1.14
Silicon has the same structure as
diamond and a bond length of 0.2351 nm. Estimate its density.
(A.U.
Jan 2010)
Given
data
In the case of diamond cubic structure,
Bond length (2r) = 0.2351 nm = 0.2351 x
10-9 m
Atomic radius (r) = 0.11755 × 10-9
m
Number of atoms per unit cell (n) = 8
[diamond structure]
Atomic weight (M) for silicon = 28.09
Avagadro's number N = 6.02 × 1026
mol-1
Solution
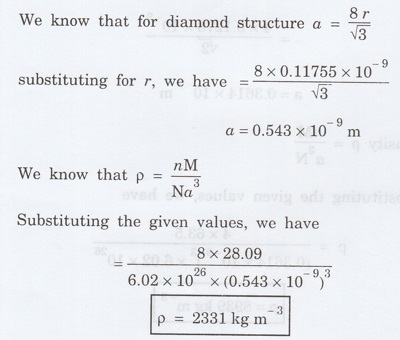
Problem
1.15
Calculate the interplanar spacing of
three important planes (100), (110) and (111) of a simple cubic system. (A.U. Jan 2013)
Solution
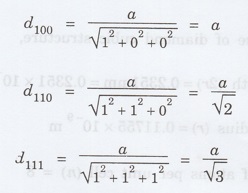
Problem
1.16
The distance between (110) plane in a
BCC structure is 2.03 Å. What is the size of unit cell?
(A.U.
Jan 2011)
Given data
d110 = 2.03 Å
h = 1, k = 1, l = 0
Solution

Problem
1.17
Show that for a simple cubic system d100:
d110: d111: :√6: √3: √2 (A.U. Jan 2013)
Solution
Distance d between the adjacent planes
is given by
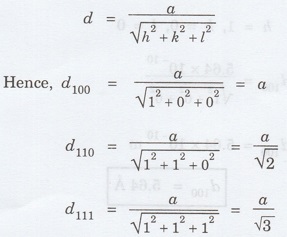
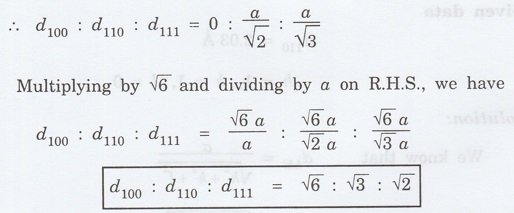
Problem
1.18
Determine spacing between (i) (100)
planes (ii) (110) planes (iii) (111) planes in NaCl crystal having a lattice
constant a = 5.64 Å. [A.U. April 2013]
Given
data
Lattice constant a = 5.64 Å = 5.64 × 10-10
m
Solution
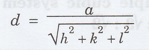
For [100] planes
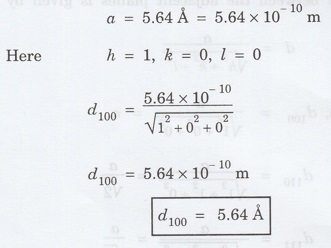
For [110] planes
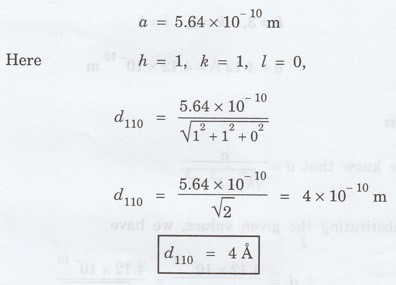
For [111] planes

Problem
1.19
Calculate the interplanar distance for
(321) plane in simple cubic lattice with interatomic spacing equal to 4.12 Å. (A.U May 2012)
Given data
h = 3, k 2, l = 1
a = 4.12 Å = 4.12 × 10-10 m
Solution

Substituting the given values, we have
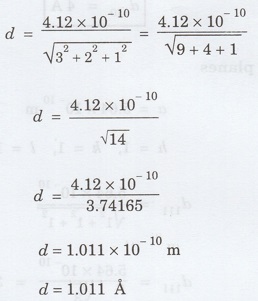
Problem
1.20
Lattice constant of copper is 0.38 nm.
Calculate the distance between (110) planes. (A.U. Jan 2013)
Given
data
a = 0.38 nm = 0.38 × 10-9 m
h = 1 k = 1 l = 0
Solution

Substituting the given values, we have

Physics for Electronics Engineering: Unit I: Crystallography : Tag: : Crystallography | Physics for Electronics Engineering - Anna University Solved Problems
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
