Physics for Electronics Engineering: Unit IV: Optical Properties of Materials
Absorption and Emission of Light in Semiconductors
In semiconductors, light photons is absorbed in several ways. In intrinsic (pure) semiconductors such as Si, Ge and GaAs, light photons is absorbed to create electron-hole pairs.
ABSORPTION AND EMISSION OF LIGHT IN SEMICONDUCTORS
In
semiconductors, light photons is absorbed in several ways. In intrinsic (pure)
semiconductors such as Si, Ge and GaAs, light photons is absorbed to create
electron-hole pairs.
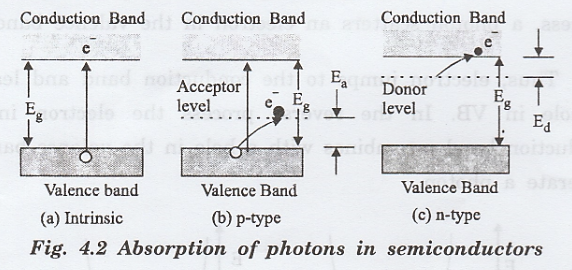
This
absorption causes electrons to jump across the energy band gap from the valence
band to the conduction band as shown in fig. 4.2(a).
This
transition occurs ie., the excitation of electrons due to absorption can take
place if the photon energy is greater than that of the band gap Eg
that is if
hv
> Eg .................(1)
where
h - Planck's constant
v
= Frequency of the light photon.
In
terms of wavelength 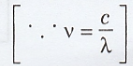

The
maximum wavelength for visible light λmax is about max 0.7 μm.
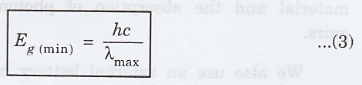
Therefore, the minimum band gap energy Eg(min) for which there is
absorption of visible light is given by
Substituting
the corresponding values, we have
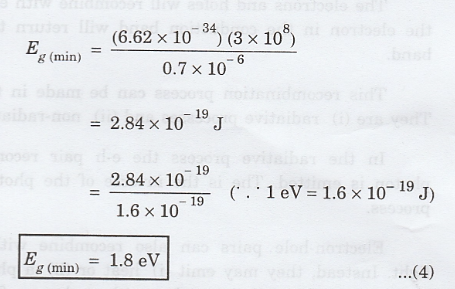
The
result indicates that all visible light is absorbed by those semiconductors
having band gap energies less than about 1.8 eV. Thus, these semiconductors are
opaque.
In
extrinsic (impure) semiconductors, the presence of acceptor and donor
impurities creates new energy levels namely acceptor level (Ea)
(p-type semiconductor) and donor level (Ed) (n-type semiconductor)
as shown in fig. 4.4 (b) and (c). These impurity levels lie within the band gap
of the material.
Light
radiation of specific wavelength may be absorbed as a result of electron
transitions from or to these impurity levels within the band gap.
Physics for Electronics Engineering: Unit IV: Optical Properties of Materials : Tag: : - Absorption and Emission of Light in Semiconductors
Related Topics
Related Subjects
Physics for Electronics Engineering
PH3254 - Physics II - 2nd Semester - ECE Department - 2021 Regulation | 2nd Semester ECE Dept 2021 Regulation
