Electronic Devices and Circuits: Unit I: Semiconductor Devices
Classification of Semiconductors
Intrinsic Semiconductor, Extrinsic Semiconductors, N-Type Semiconductor, P-Type Semiconductor
Some valence electrons may acquire sufficient energy to enter the conduction band to form free electrons such electrons constitute current when electric field is applied.
SEMICONDUCTORS
Semiconductors
are classified as
i. Intrinsic Semiconductors
ii.
Extrinsic Semiconductors
Intrinsic Semiconductor
A
semiconductor in its purest form is called intrinsic semiconductor.
i.
Some valence electrons may acquire sufficient energy to enter the conduction
band to form free electrons such electrons constitute current when electric
field is applied.
ii.
The missing electron creates a vacant space which is known as hole. Thus, even
at room temperature electron-hole pairs are created.
iii.
When electric field is applied, the current flows due to free electrons and
holes.
Extrinsic Semiconductor
To
increase the amount of current flow in semiconductor, a small amount of
impurity is added to the intrinsic semiconductor such type of semiconductor is
called extrinsic semiconductors.
i.
The process of adding impurity to intrinsic semiconductor is known as doping.
The added impurity is called dopant.
ii.
The amount of doping is such that 1 to 2 impurity atoms are added to 106
intrinsic atoms.
iii.
There are two types of impurity atoms
a.
Pentavalent atom
b.
Trivalent atom
iv.
The pentavalent atom donates one free electron to an intrinsic material. So
this type of impurity is called donor impurity.
Example:
Phosphorous, Arsenic, Antimony
v.
The trivalent atom has only three valence electrons, it creates more holes and
accepts an electron. So, this impurity is called acceptor impurity.
Example:
Boron, Gallium, Indium
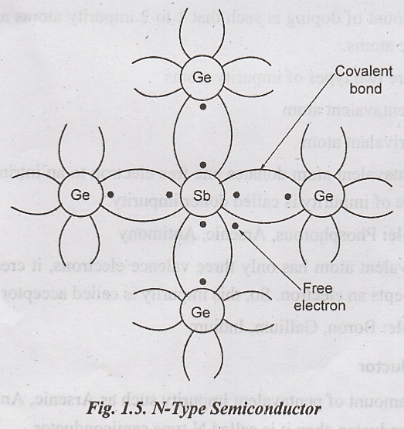
N-Type Semiconductor
When a small amount of pentavalent impurity such as Arsenic, Antimony is added to the pure semiconductor, then it is called N-type semiconductor.
Germanium
has four valence electron and Antimony has five valence electron. Each Antimony
atom forms a covalent bond with surrounding four Germanium atoms.
The
fifth valence electron of Antimony atom is loosely bound to the Antimony atom.
i.
If electric field is applied, then it can be easily excited from the valence
band to the conduction band.
ii.
Thus pentavalent impurities donate one electron for conduction. So it is called
donor impurity.
iii.
After losing one electron, the donor atom becomes positively charged ion and it
cannot conduct.
iv.
Thus, adding pentavalent impurity increases the number of electrons in the
conduction band and hence the conductivity increases.
v.
In N-type material, the numbers of electrons are more than that of holes.
Therefore, the electrons are called majority carriers and the holes are called
minority carriers. The conduction is mainly due to electrons.
P-Type Semiconductor
When
a small amount of trivalent impurity is added to pure semiconductor, it is
called P-type semiconductor.
i.
The trivalent impurity such Boron has three valence electrons. These electrons
form three covalent bonds with the surrounding Germanium atom.
ii.
The one remaining covalent bond cannot be formed because Boron has only three
valence electrons and Germanium has four valence electrons.
iii.
This creates a hole in the place of incomplete covalent bond.
iv.
Thus large number of holes is present in the valence band due to doping.
v.Trivalent
impurity is also called acceptor atom because it accepts free electrons in the
place of holes.
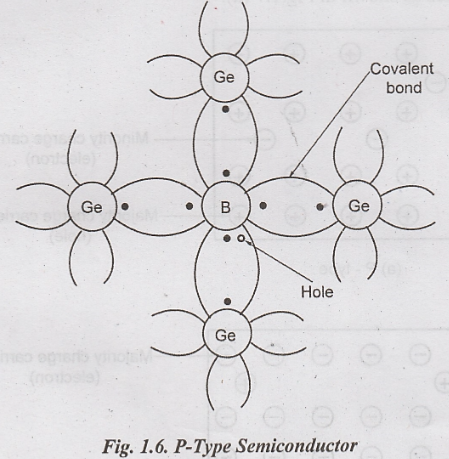
vi.
When each boron atom donate a hole for conduction, it becomes negatively
charged ion.
vii.
The number of holes is greater than that of free electrons in P-type material.
viii. The conduction is mainly due to holes. Hence holes are called majority carriers and electrons are called minority carriers.
Electronic Devices and Circuits: Unit I: Semiconductor Devices : Tag: : Intrinsic Semiconductor, Extrinsic Semiconductors, N-Type Semiconductor, P-Type Semiconductor - Classification of Semiconductors
Related Topics
Related Subjects
Electronic Devices and Circuits
EC3353 - EDC - 3rd Semester - ECE Dept - 2021 Regulation | 3rd Semester ECE Dept 2021 Regulation
